
Melting Point, Boiling Point, and Heat of Vaporization of Some Common... | Download Scientific Diagram

Approximated latent heat of vaporization for (a) butane; (b) isobutane;... | Download Scientific Diagram
I. Latent heat of vaporization of n-hexane; II. Volumetric and latent heat of vaporization measurements for trans-2-butene; III. Partial enthalpy change upon vaporization for n-butane in the n-butane-n-decane system - CaltechTHESIS

vapor pressure - Why is latent heat of vaporization not exactly proportional to boiling point? - Chemistry Stack Exchange

How much energy is required to vaporize 155 g of butane at its boiling point? the heat of - Brainly.com
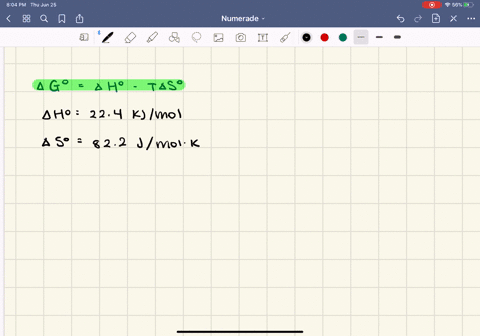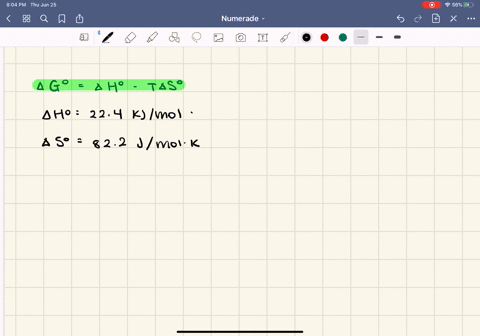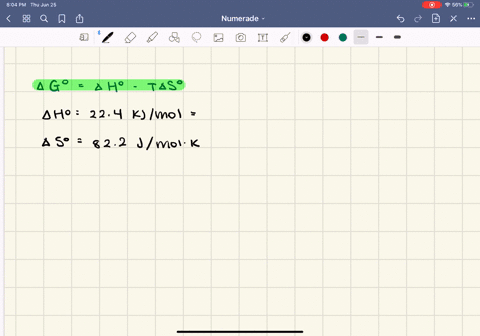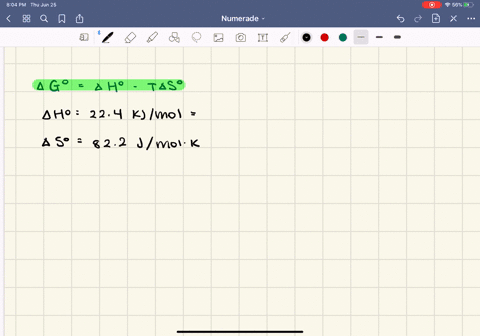
SOLVED:The enthalpy of vaporization of butane, \mathrm{C}_{4} \mathrm{H}_{10}, is 22.44 \mathrm{kJ} / \mathrm{mol} , and the entropy of vaporization is 82.2 \mathrm{J} / \mathrm{mol} \cdot \mathrm{K} .

The enthalpy of fusion of solid n-butane is 4.66 kJ/mol. Calculate the energy required to melt 58.3 g of solid n-butane. | Homework.Study.com

Entropy | Free Full-Text | Optimization of the Changing Phase Fluid in a Carnot Type Engine for the Recovery of a Given Waste Heat Source | HTML
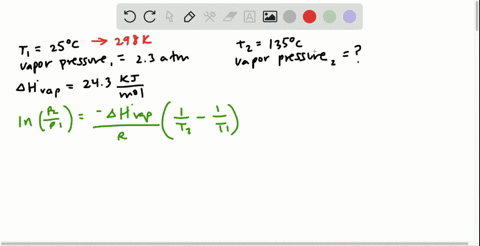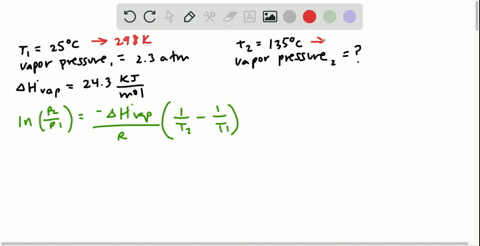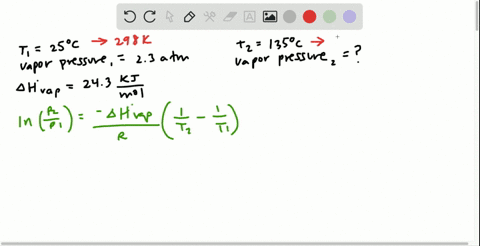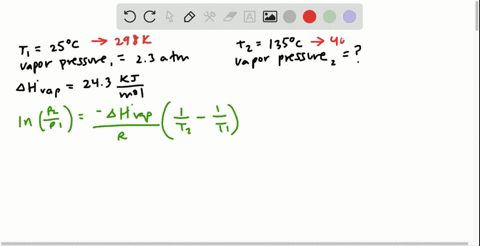
SOLVED:Butane (C4H10) has a heat of vaporization of 22.44 kJ>mol and a normal boiling point of -0.4 C. A 250.0 mL sealed flask contains 0.55 g of butane at -22 C. How
Problem Set #10 Assigned November 8, 2013 – Due Friday, November 15, 2013 Please show all work for credit To Hand in 1.

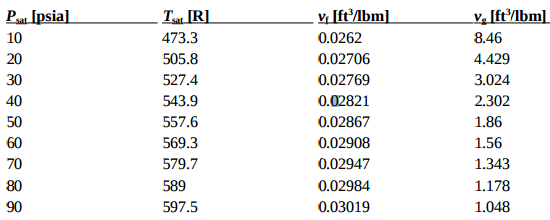
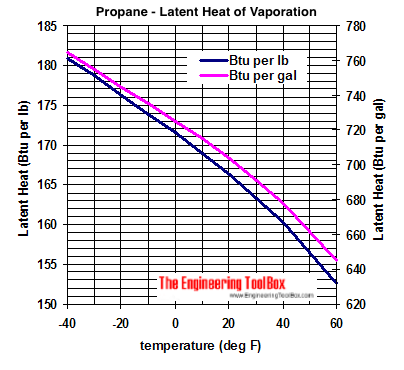
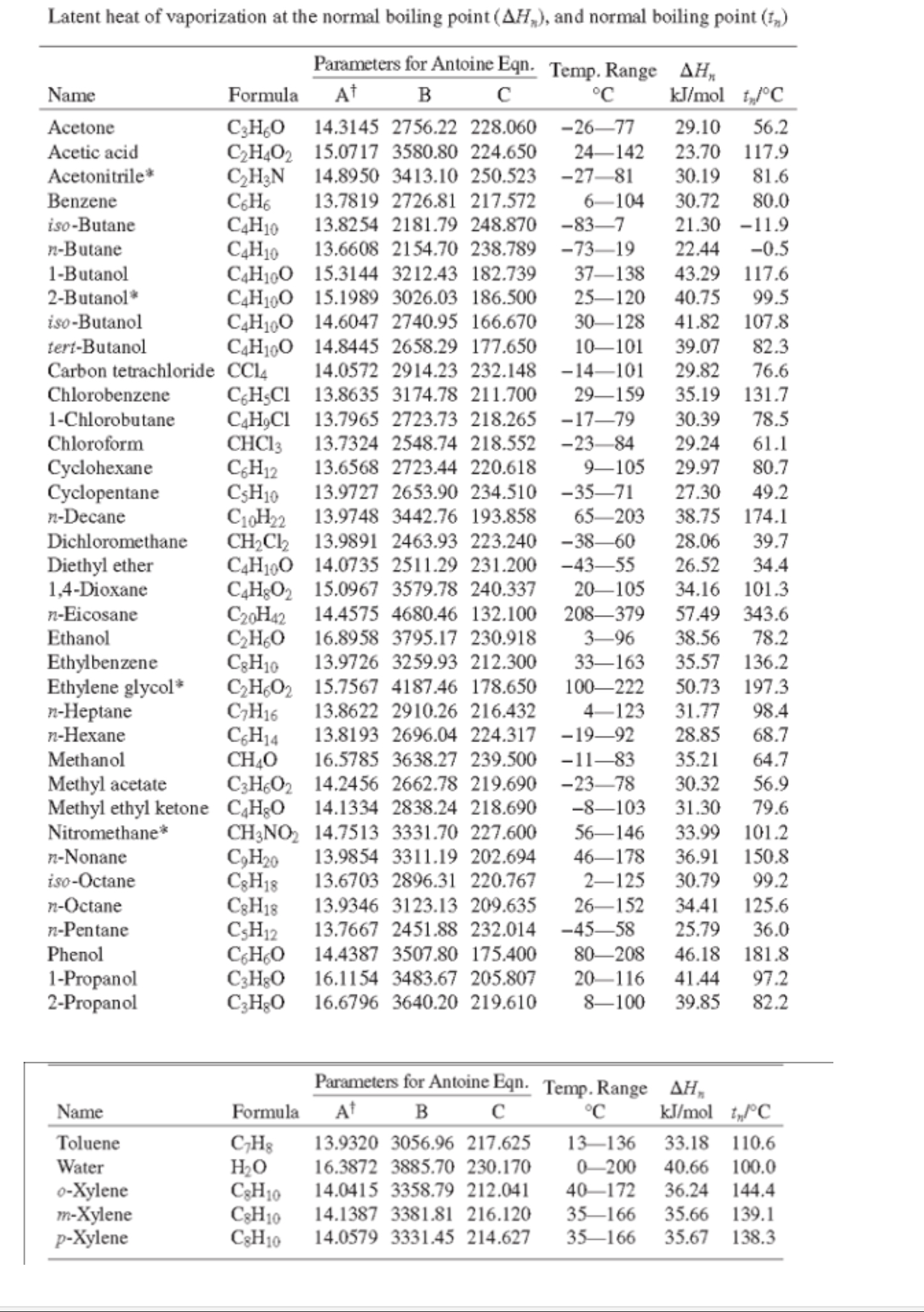

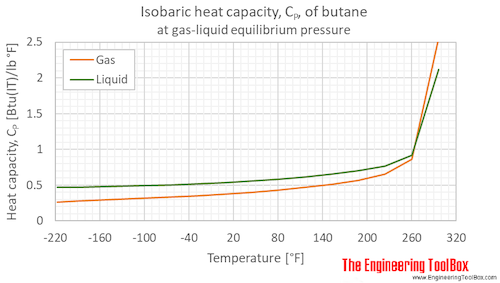


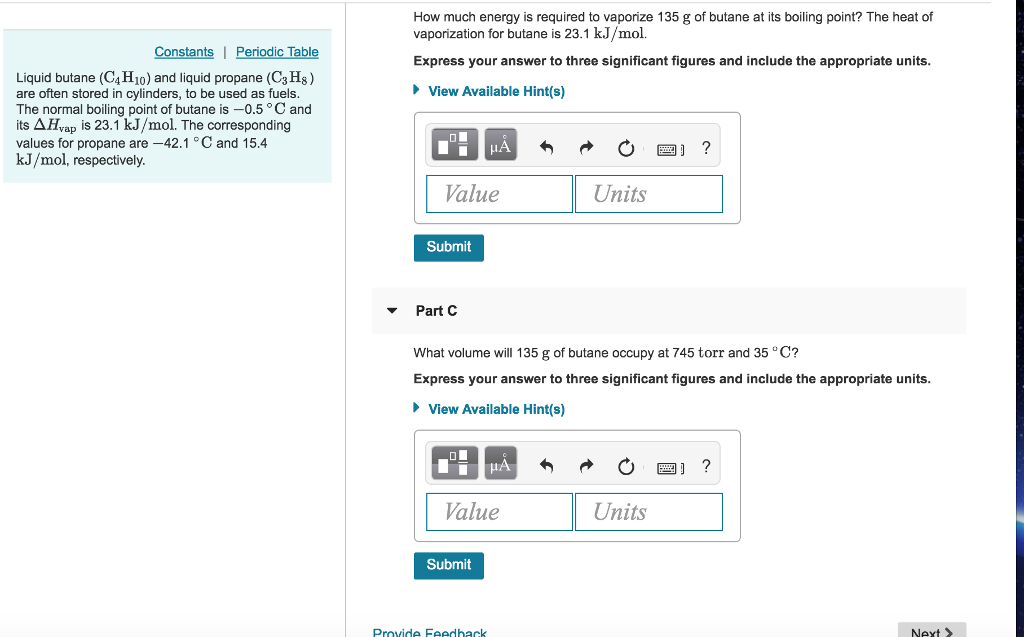
![Latent heat of vaporization for main components of LNG [10]. | Download Table Latent heat of vaporization for main components of LNG [10]. | Download Table](https://www.researchgate.net/publication/330572654/figure/tbl3/AS:718422421803010@1548296661881/Latent-heat-of-vaporization-for-main-components-of-LNG-10.png)

