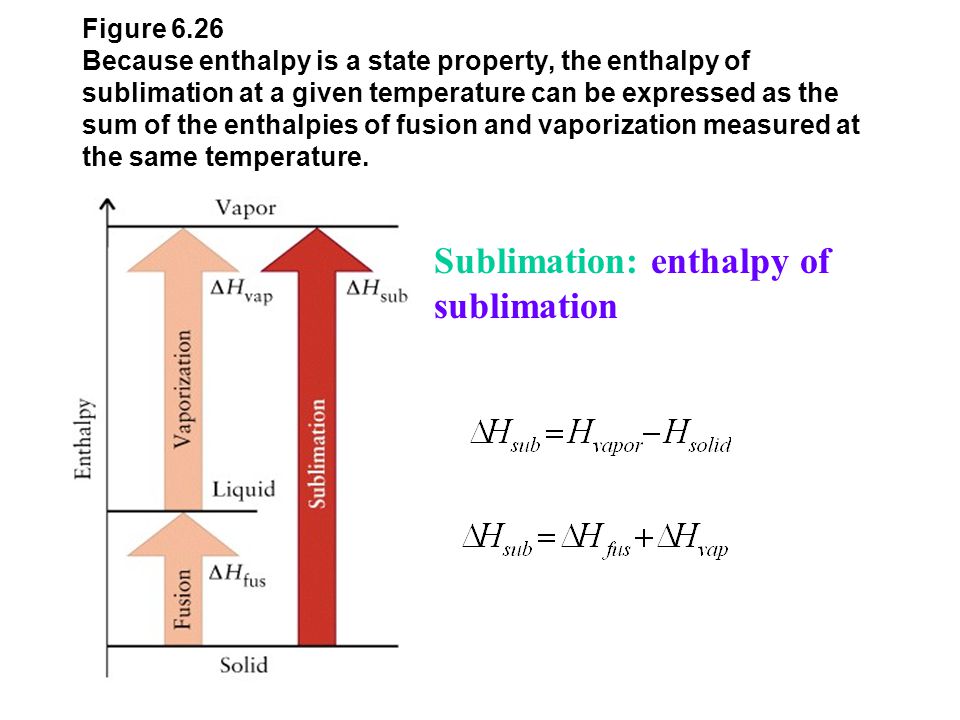
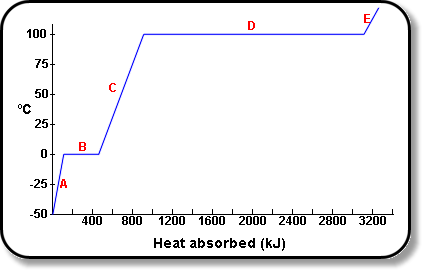
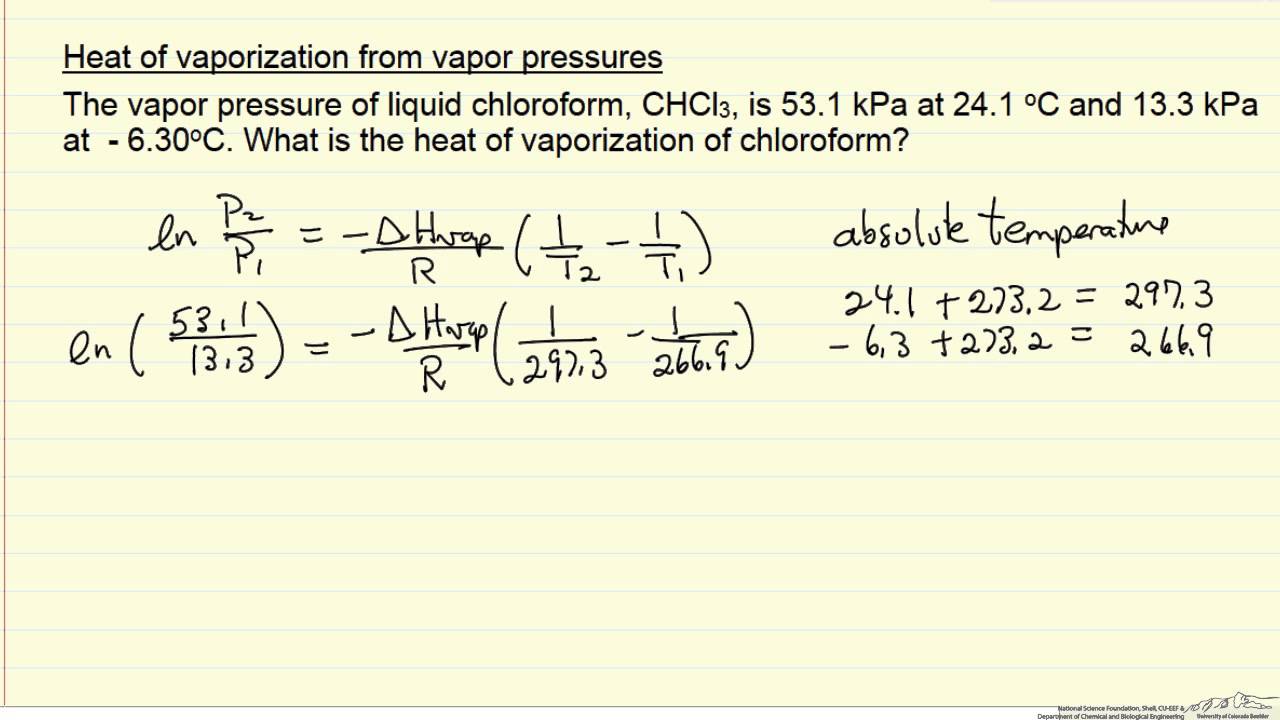
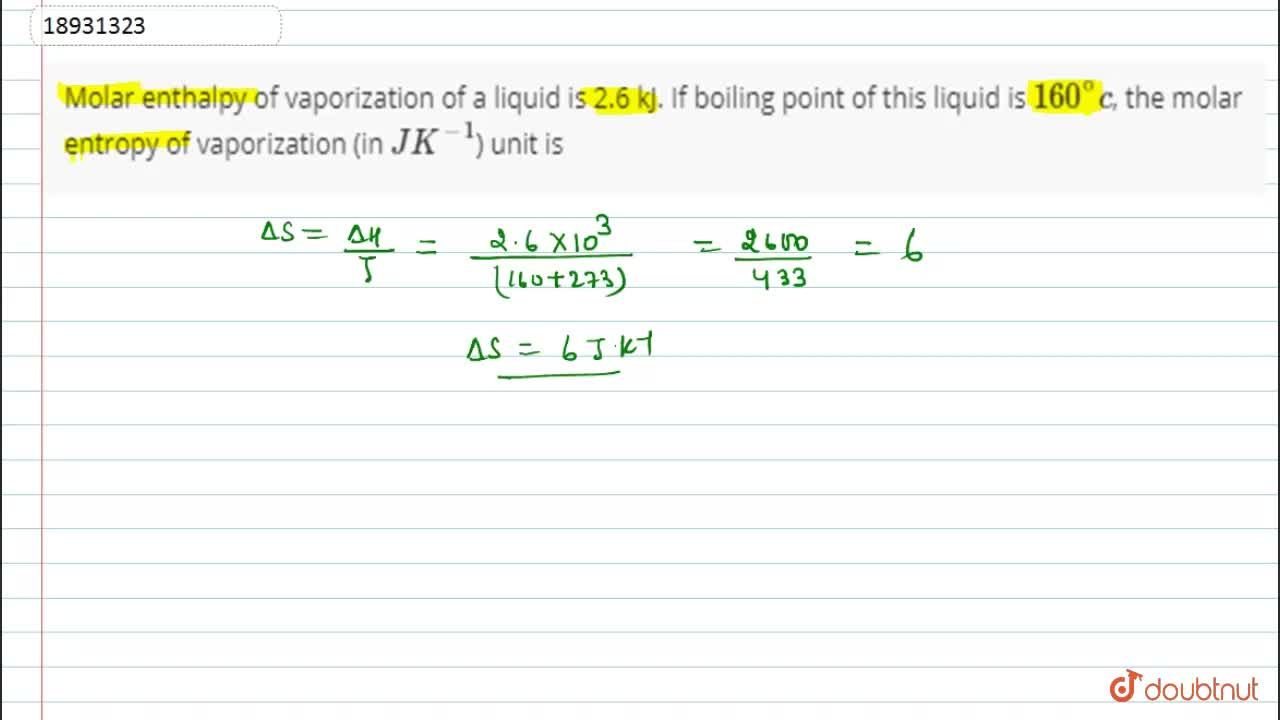
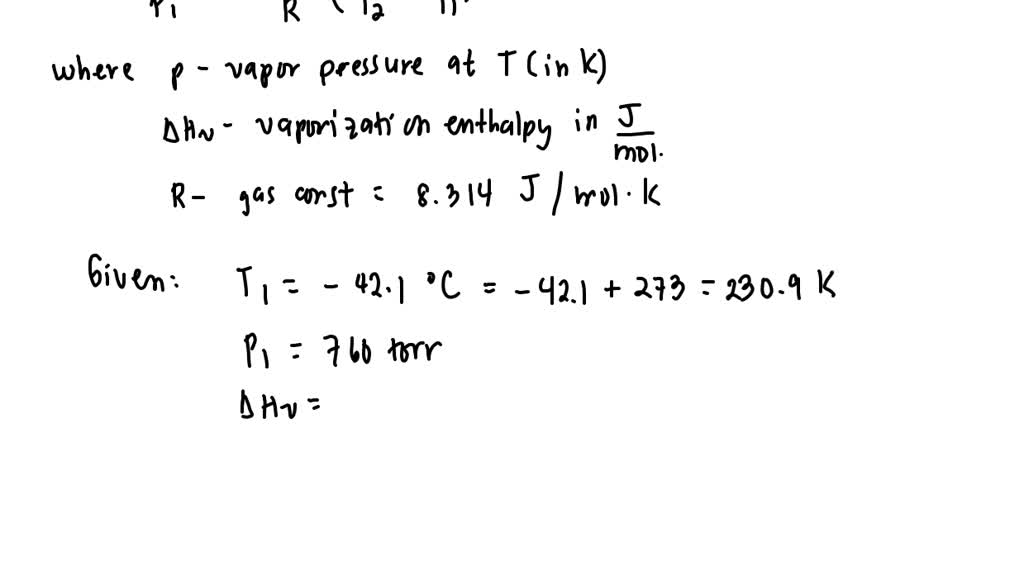Calculate the molal elevation constant of water if molar enthalpy of vaporisation of water at 373 K is 40.585 kJ/mol.

Calculate the enthalpy of vaporisation per mole for ethanol. Given, Δ S = 109.8JK^-1mol^-1 and boiling point of ethanol is 78.5^oC .

SciELO - Brasil - Enthalpy of mixing and heat of vaporization of ethyl acetate with benzene and toluene at 298.15 k and 308.15 k Enthalpy of mixing and heat of vaporization of

Describe (qualitatively) how standard enthalpy and entropy of vaporization of water will change with temperature. | Homework.Study.com

Ethanol has a heat of vaporization of 38.56 kj/mol and a normal boiling point of 78.4 C - Home Work Help - Learn CBSE Forum