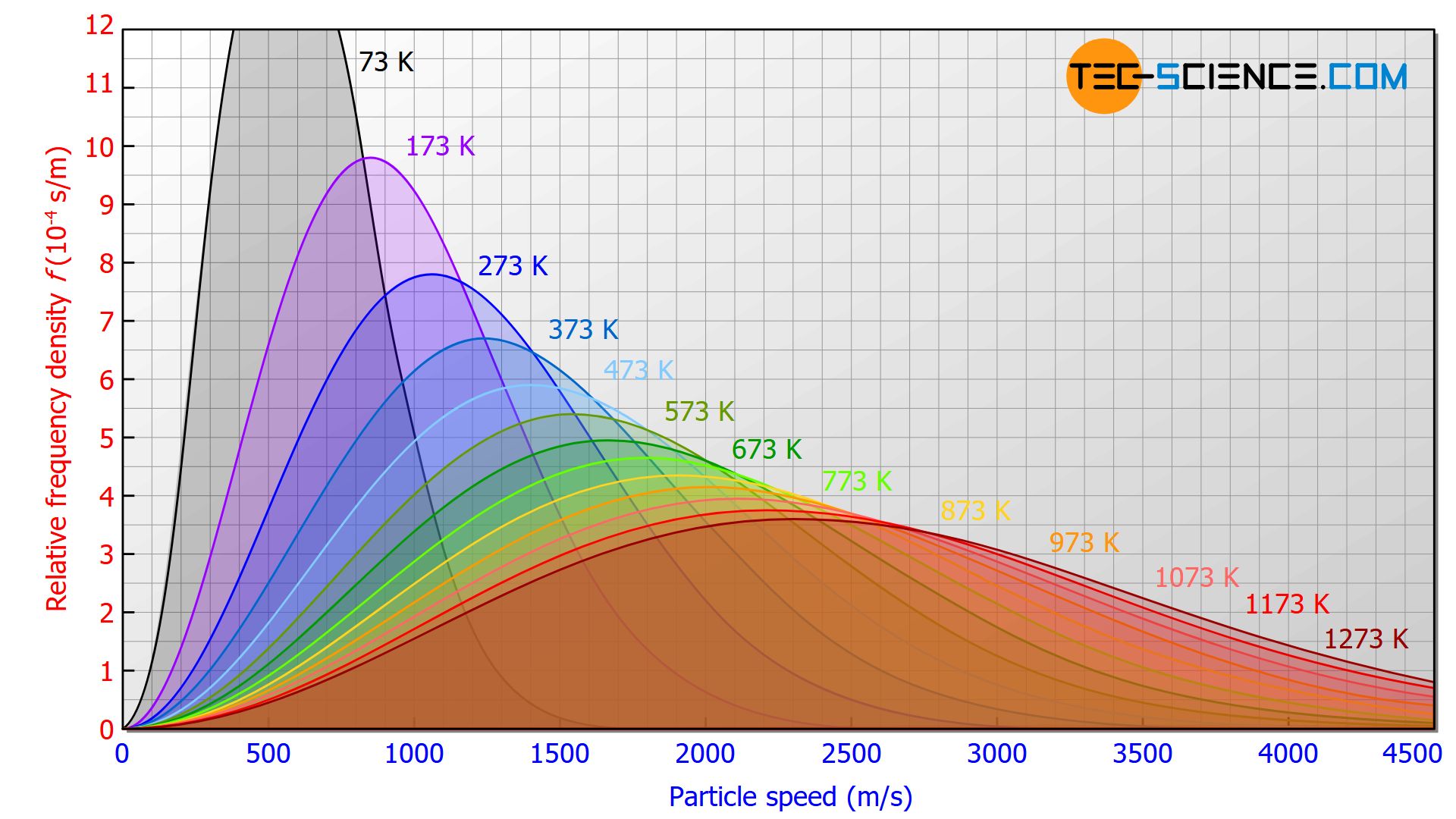
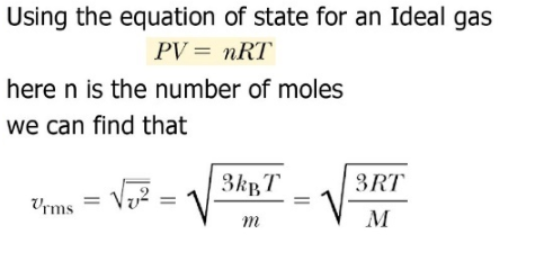
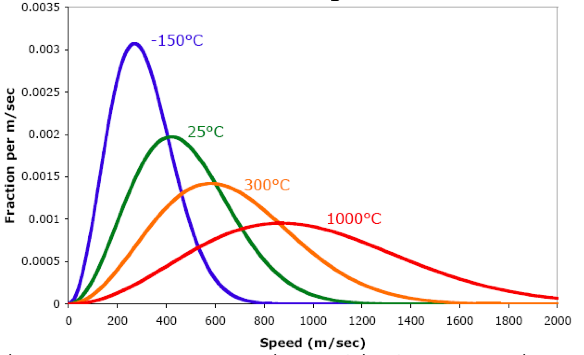
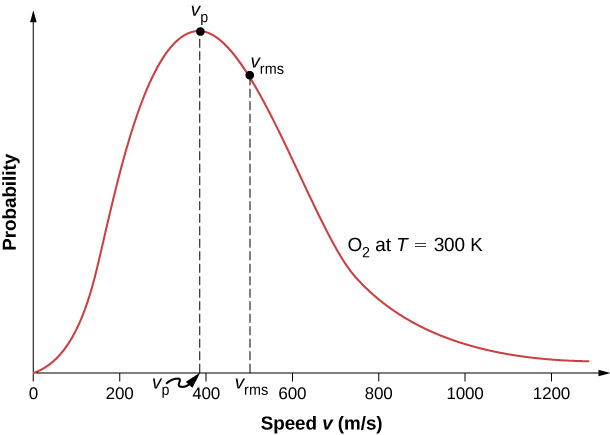
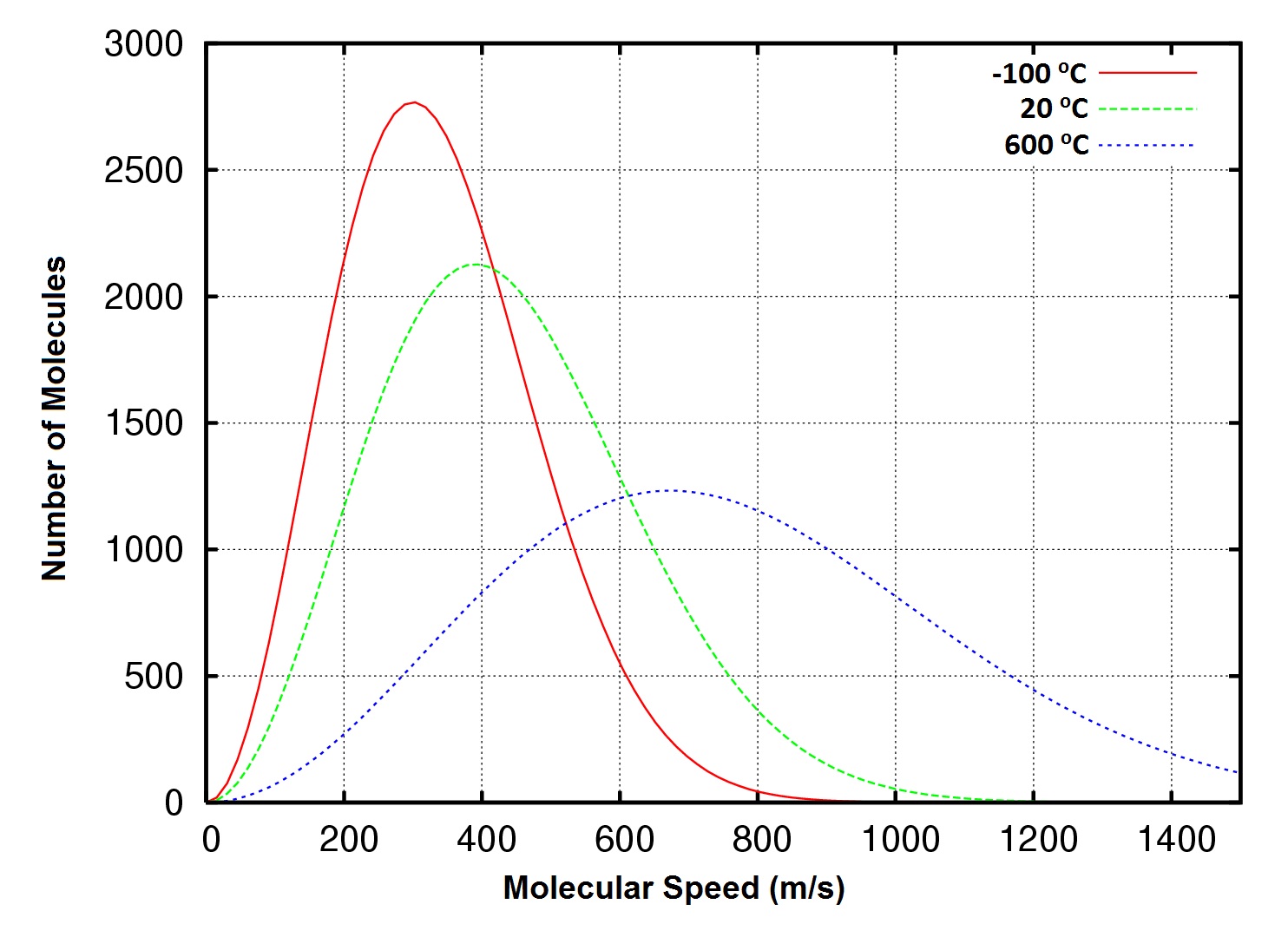
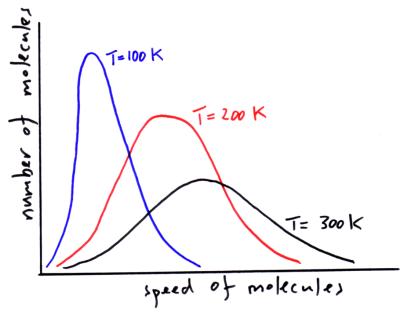
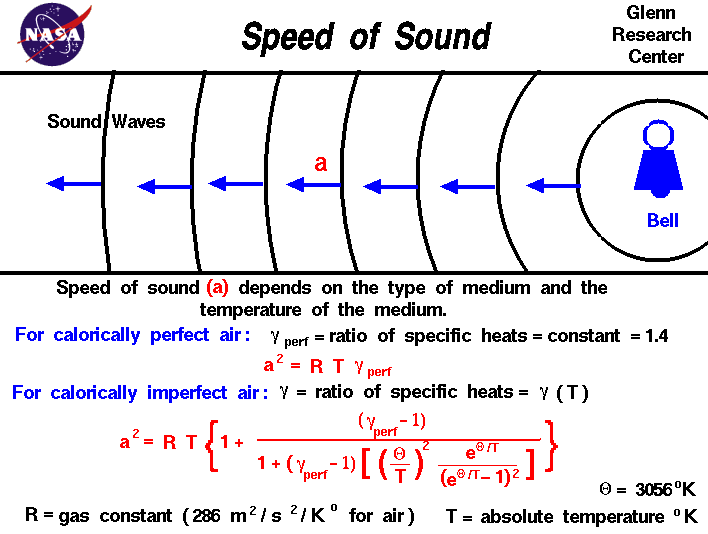
At what temperature, the average speed of gas molecules be double of that at temperature, `27^(@)C`? - YouTube

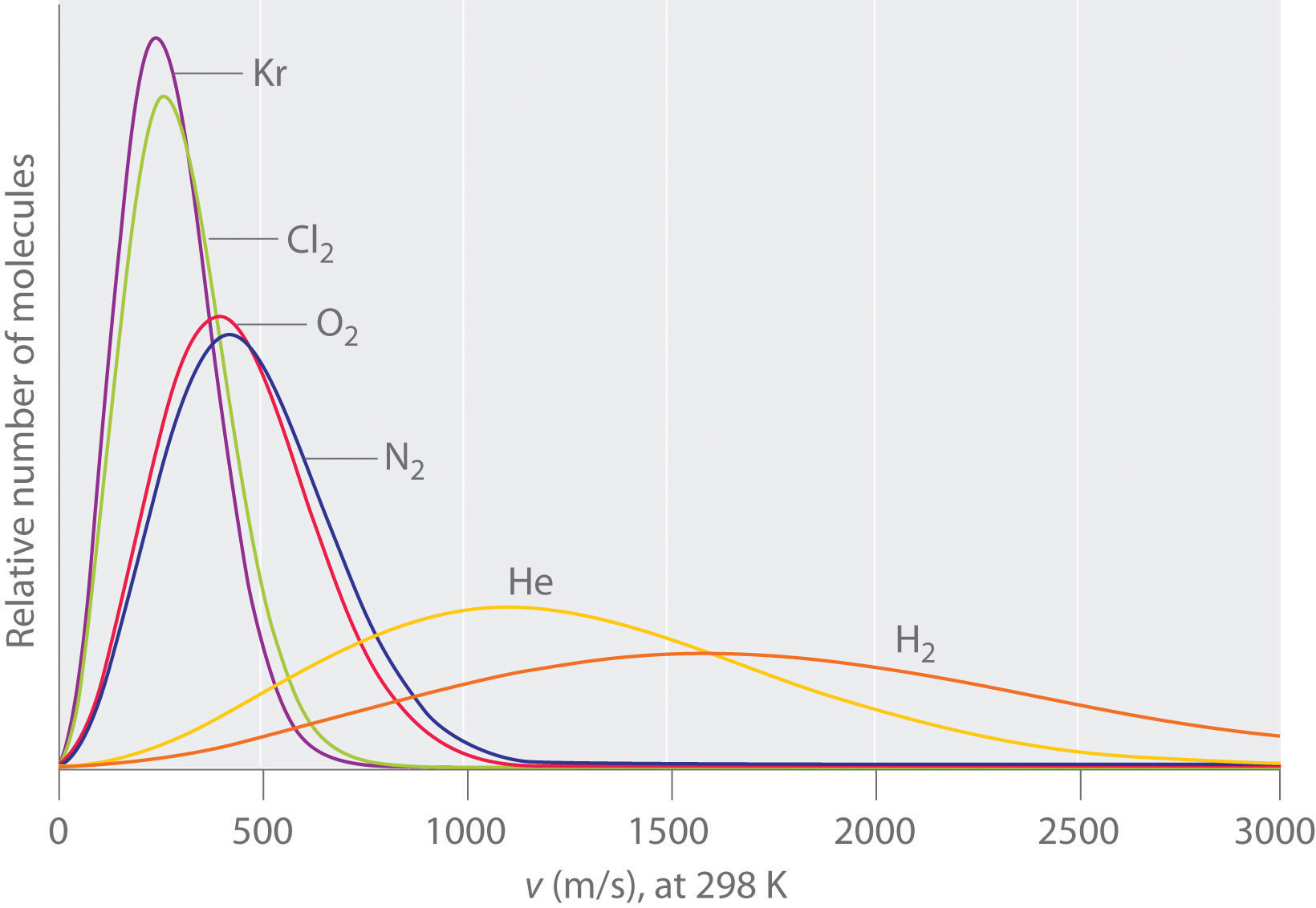
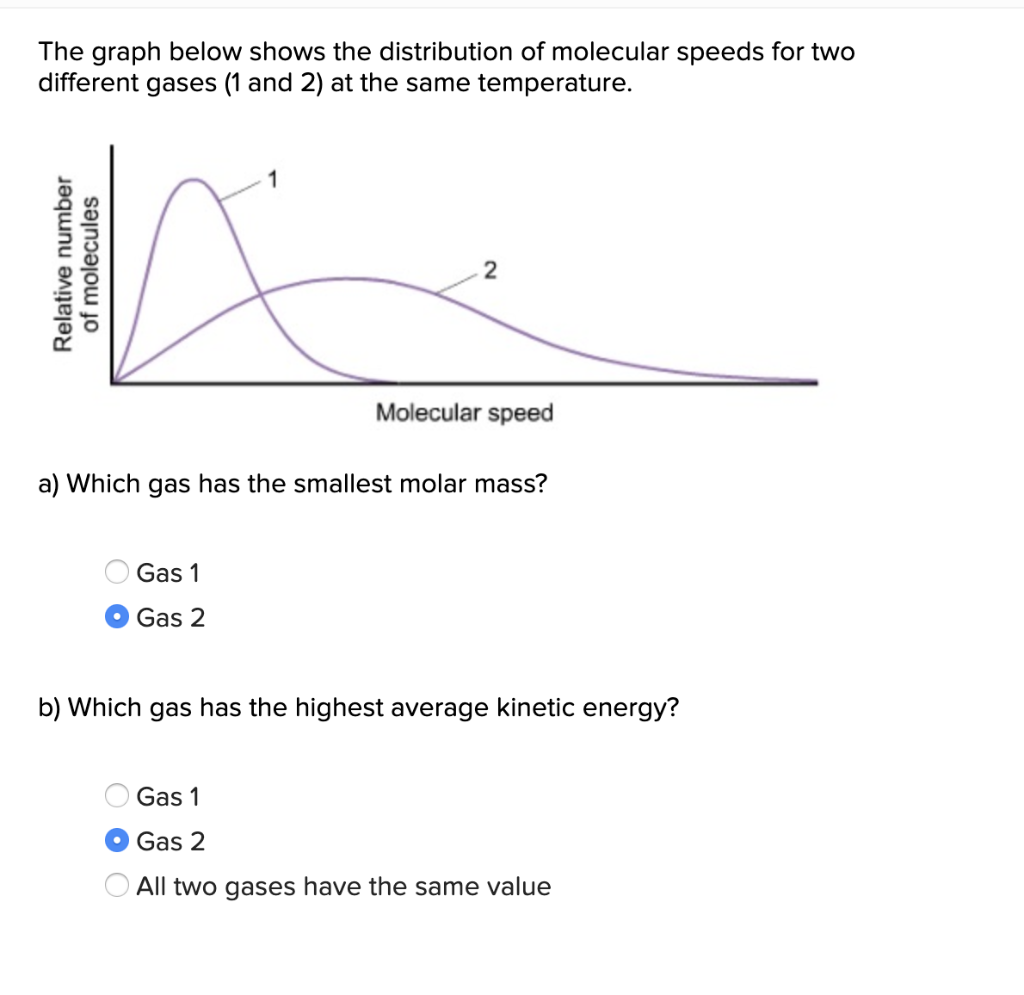
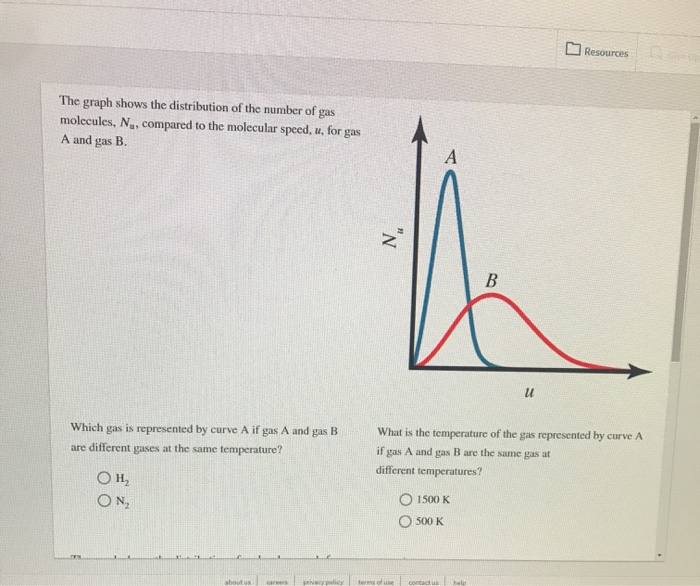
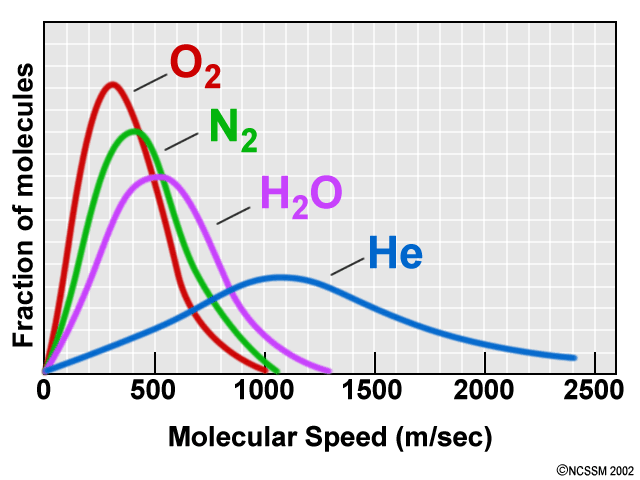
The graph below shows the relative speeds of various gases. at the same temperature. Select all true statements. A. Gas D is expected to have the lowest molar mass. B. Gas A

Kinetic Molecular Theory of Gases and Root-Mean-Square Speed (Calculating gas KE/speed) | Root mean square, Chemistry basics, Molecular
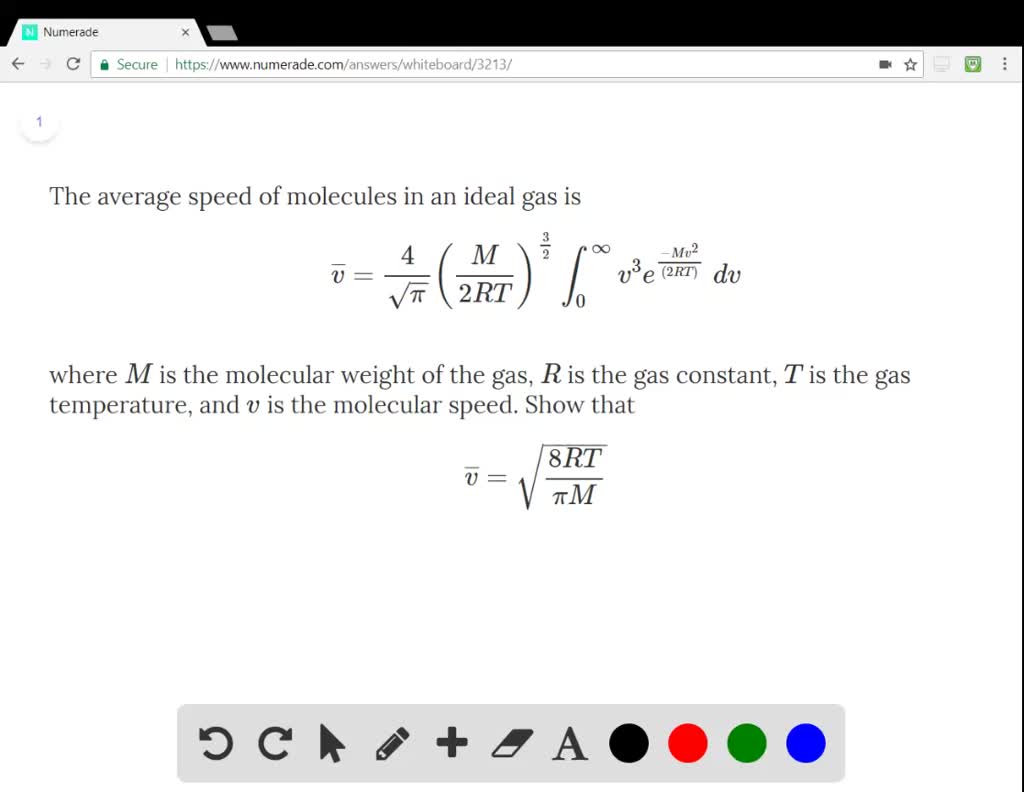
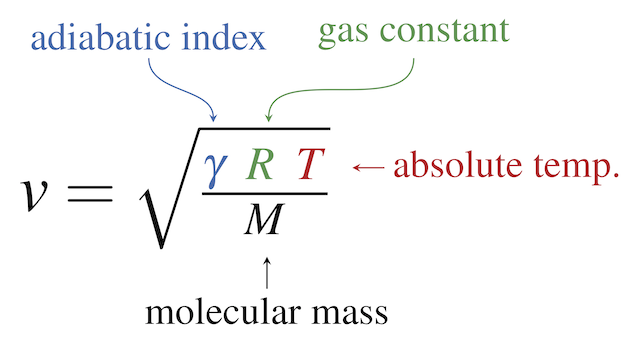
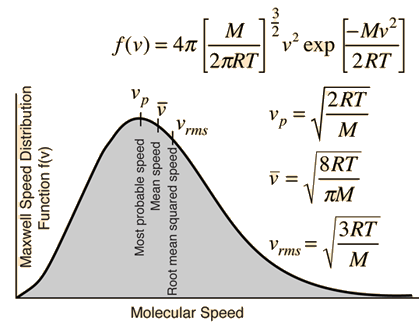
SOLVED:The average speed of molecules in an ideal gas is \overline{v} = \frac{4}{\sqrt{\pi}} \left (\frac{M}{2RT} \right)^{\frac{3}{2}} \int_0^\infty v^3 e^{\frac{-Mv^2}{(2RT)}}\ dv where M is the molecular weight of the gas, R is the