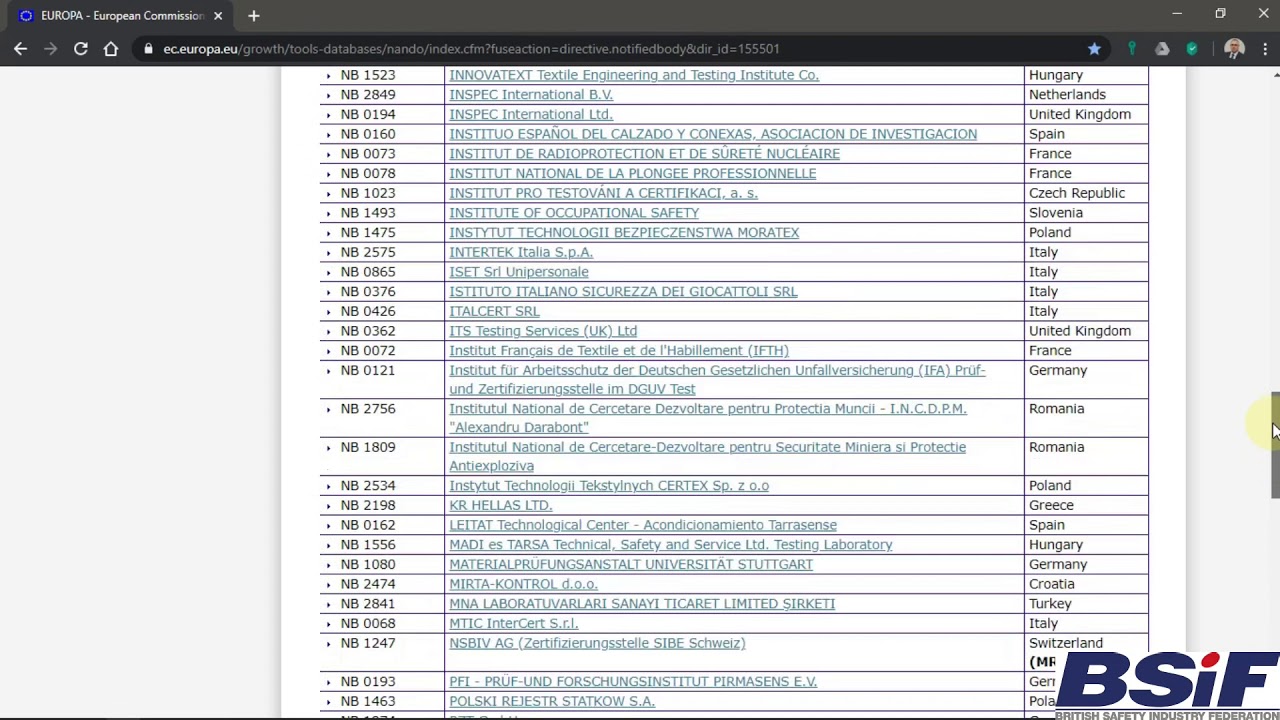
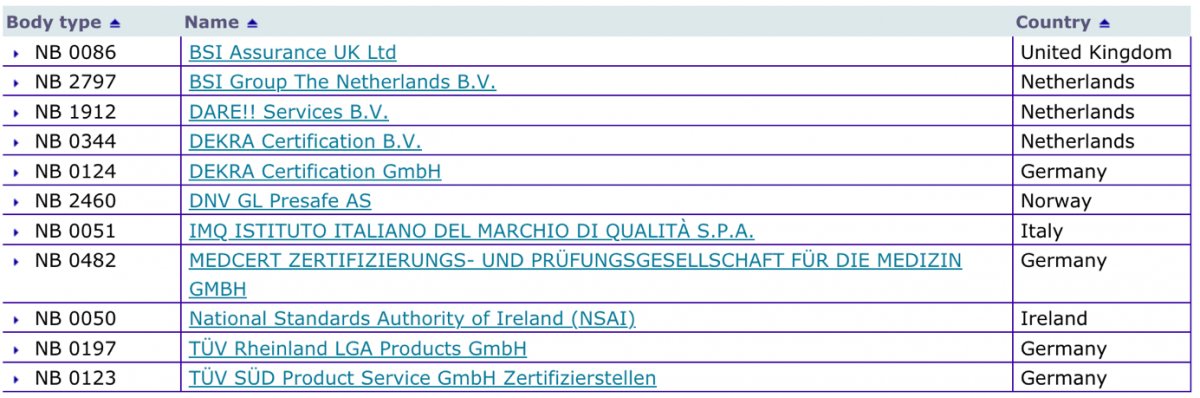
Informational - New designated Notified Body for Regulation (EU) 2017/745 ( MDR) included in Nando – IMQ ISTITUTO ITALIANO DEL MARCHIO DI QUALITÀ S.P.A.
Low rate of Notified Body designations under MDR and IVDR causes bottleneck concerns for the European MedTech Industry

MDR: 29 Notified Bodies on NANDO · MDlaw – Information platform on European medical device regulations