At one bar pressure, the volume of a gas is 0.6 liter. If the gas receives 122 Joules of heat at one atmosphere pressure, the volume becomes 2 liters, then calculate its
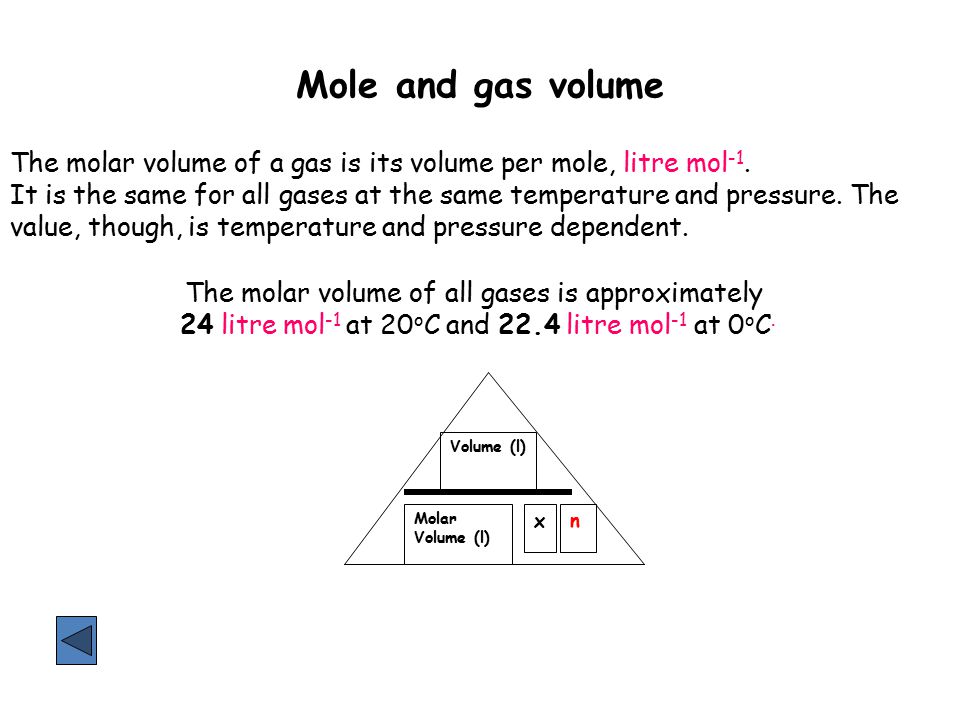
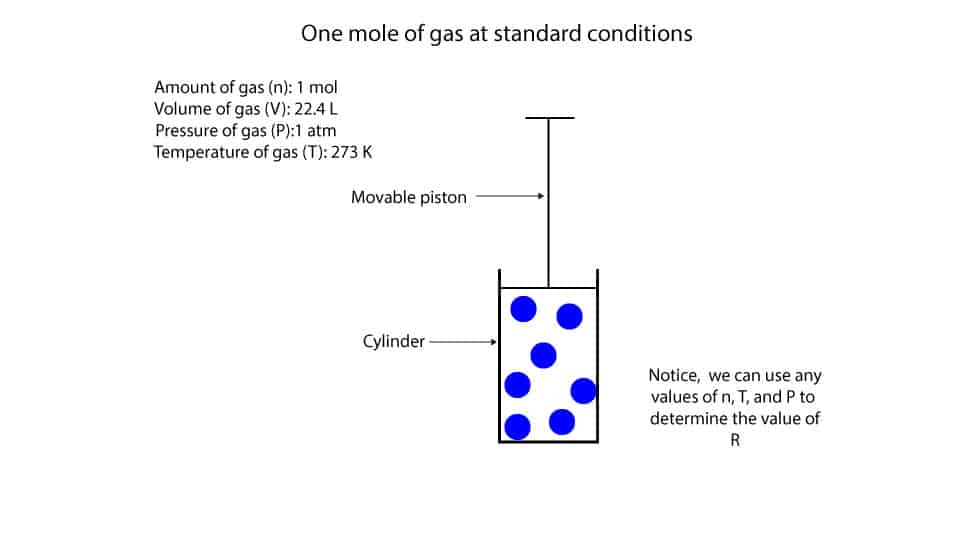
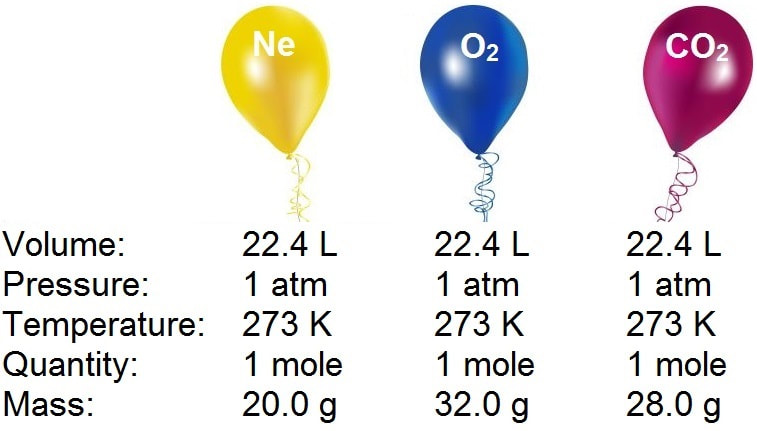
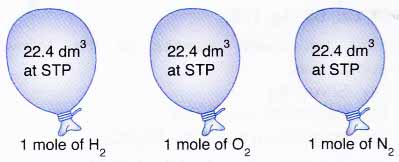
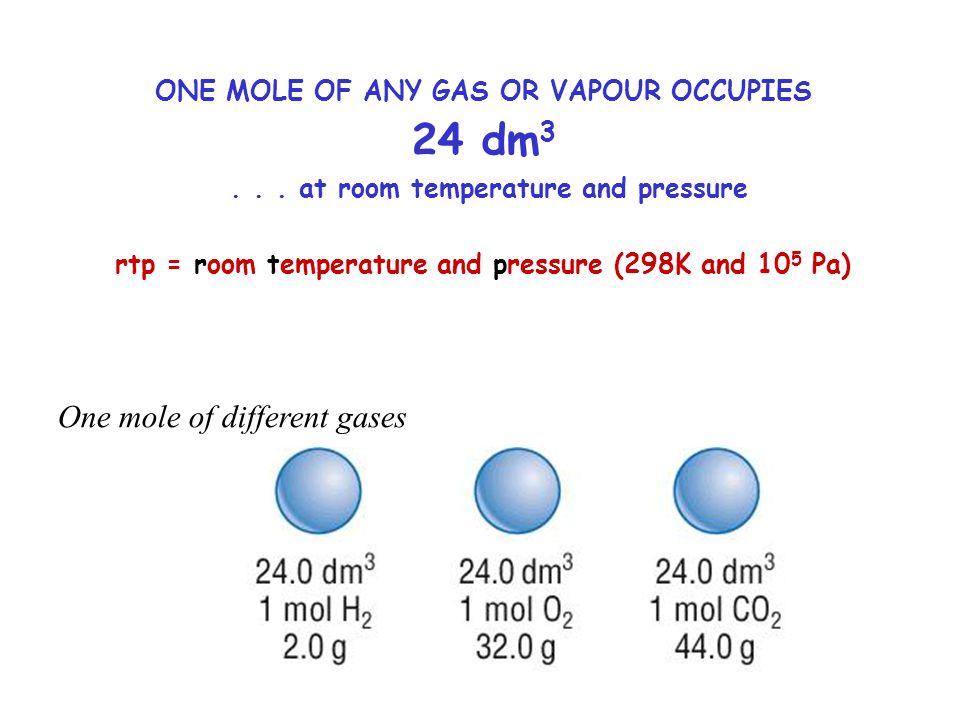
Mole and gas volume The molar volume of a gas is its volume per mole, litre mol-1. It is the same for all gases at the same temperature and pressure. The. -

One mole of an ideal gas at standard temperature and pressure occupies 22.4 L(molar volume). What is the ratio of molar volume to the atomic volume of a mole of hydrogen ? (

1 mole of gas occupies a volume of 100 ml at 50 mm pressure . What is the volume occupied by two - YouTube

Molar volume is the volume occupied by 1 mole of any (Ideal) gas at standard temperature and pressure (STP , 0^(@) C, 1 atmospheric pressure). Show that it is 22.4 litres. Take R = 8.31 J mol^(-1) K^(-1).
One mole of an ideal gas at STP occupies 22.4 L. What is the ratio of molar volume to atomic volume of a mole of hydrogen? - Sarthaks eConnect | Largest Online Education Community
Solved] Assume that one mole of gas occupies a volume of 24.5 L at room temperature and 1 atm. You mix 0.05 moles of sodium bicarbonate and 0.01 mol... | Course Hero
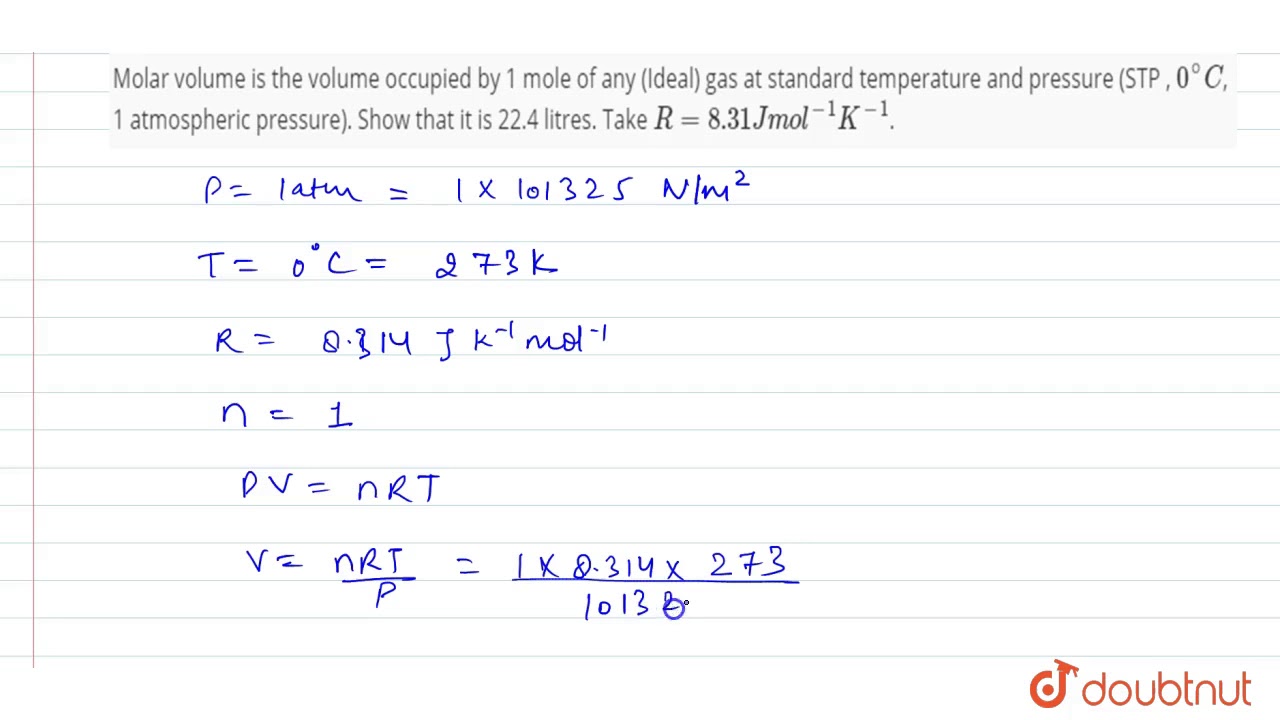
Molar volume is the volume occupied by 1 mole of any (Ideal) gas at standard temperature and pre... - YouTube

Volume of one mole gas changes according to the V = aT . If temperature change is Δ T , then work done will be

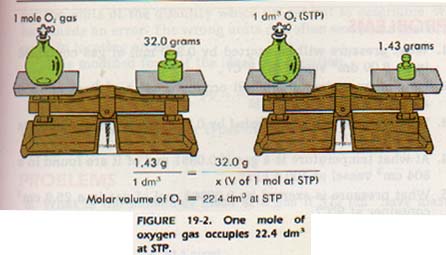
What volume will 1 mole of a gas occupy at STP? STP = 273K, 1.013x10 5 Pa One mole of any ideal gas occupies a volume of 22.4L at STP. - ppt download

1 mole of gas occupies a volume of 100 ml at 50 mm pressure. What is the volume occupied by two moles of gas at 100 mm pressure and at same temperature